Quality Control
PALTAC handles a wide range of products, including Cosmetics, Daily necessities, and OTC pharmaceuticals. Our social mission is to deliver products that can be used with peace of mind by conducting pharmaceutical affairs management to ensure the "quality, efficacy, and safety" of pharmaceuticals and other "life-related products". To this end, we are committed to thorough quality control from the manufacturer to the retailer.
Date Control
-
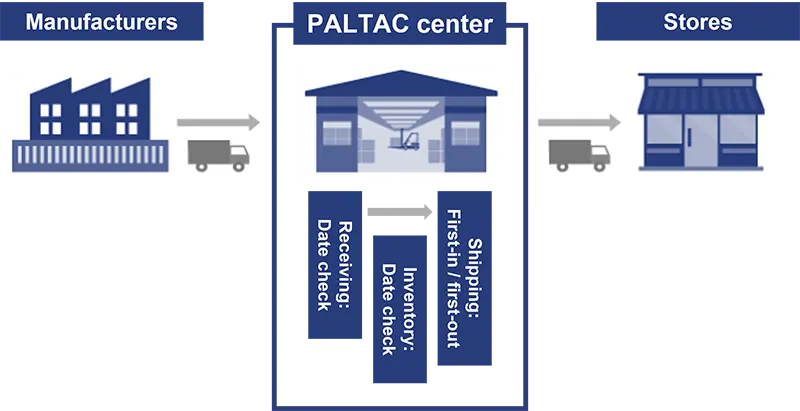
PALTAC checks the expiration dates of pharmaceuticals, quasi-drugs, health foods, and other products with use-by and best-before dates upon receipt and registers them as data for date management in our system. In principle, product shipments are made on a first-in, first-out basis. The system checks on a daily basis for products that are approaching their internally set shipping deadlines and processes products that are about to expire to exclude them from shipments, thereby ensuring date and quality control so that retailers can sell them with peace of mind.
In addition, we are promoting the digitization of logistics information between multiple manufacturers and our company. By automatically reflecting expiration date information added to ASN data (advance shipment information) received from manufacturers, we are reducing the work required to input the dates of expiration date-controlled products at the time of arrival. -
Quality Control Based on GDP Guidelines and JGSP
Quality control for storage and distribution of pharmaceuticals is based on the GDP Guideline*1 and JGSP <Generic Drug Version>*2. We have prepared various manuals and procedures for logistics-related operations and pharmacist management, etc., and focus on establishing appropriate storage and distribution systems based on these manuals and procedures.
- GDP Guideline (Good Distribution Practice: Good Distribution of Pharmaceuticals): This defines methods to ensure that control of distribution channels (purchasing, storage, supply) is guaranteed and that the integrity of pharmaceuticals is maintained, as well as appropriate methods to prevent the flow of counterfeit pharmaceuticals into legitimate distribution channels.
- JGSP (Japanese Good Supplying Practice): The JGSP (Japanese Good Supplying Practice) is a code of practice for the supply and quality control of OTC pharmaceuticals. It is a voluntary code of practice for the pharmaceutical wholesale industry established by the Japan Pharmaceutical Wholesalers Association based on pharmaceutical-related laws and regulations.
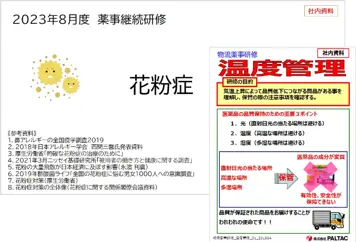
Continuing Training in Pharmaceutical Affairs
-
We provide ongoing training for sales representatives and pharmacists to develop their knowledge and qualifications to properly collect and provide information on pharmaceuticals and medical devices. For sales representatives, we provide training and education through Branch Office pharmacists on not only the efficacy of drugs, but also on pathophysiology, the Pharmaceuticals and Medical Devices Act and other related laws and regulations to understand them. We also provide training for pharmacists to enlighten them with professional and academic materials and to share information.
-